Premiere Aveta Webinar | Meditrina Presents: The Good, the Bad, and the Ugly of Hysteroscopic Systems
Join three experienced hysteroscopists, Dr. Peter Heinlein, Dr. Zoyla Almeida, and Dr. Erica Stockwell, as they share comparative insights from extensive real-world use of leading hysteroscopic systems such as, Aveta, MyoSure, TruClear, Symphion, and traditional resectoscopes.
Moderated by Dr. Peter Heinlein.
Through discussion of case experiences and procedural nuances, these three expert speakers will highlight differences in visualization, tissue removal efficiency, ergonomics, and patient comfort. Attendees will gain a deeper understanding of system selection and optimization for all procedural settings. This session is designed for gynecologists and women’s health professionals seeking practical, experience-based perspectives on current hysteroscopic technologies.
Myomectomy using the Aveta System is shown and narrated by Dr. Erica Stockwell, Advent Health FL.
Find Out Why Byron Hapner, DO, and His Inspira Colleagues Switched to Aveta
Find out why Byron Hapner, DO, and his Inspira Medical Center Mullica Hill colleagues switched to Aveta for all their diagnostic and therapeutic hysteroscopies, including calcified myomectomies. Watch the video to learn about some of the many Aveta features that can benefit gynecologic surgeons. Schedule a trial today by contacting us at info@meditrina-inc.com.
Dr. Byron Hapner, DO, removes a calcified fibroid using the Aveta Hysteroscopy System.
Aveta | the System that puts You in Control
The Aveta System brings precise control to your hysteroscopy procedures. Its physician-controlled scope lets you take HD images, adjust pressure and fluid deficit, enable irrigation and on-demand rapid flush, and control fluid management, all from the handle. With a head-up display, all the important information you need is always right in front of you. With Aveta, you can focus on what’s most important, your patient, without having to look away to search for help if resources are limited.
Dr. Geoffrey Bowers Axia Women’s Health Resects 2cm Partially Calcified Fibroid | Aveta
Watch Dr. Geoffrey Bowers with Axia Women's Health South Jersey, resect a 2cm partially calcified myoma using the Aveta System. Schedule a trial today by contacting us at info@meditrina-inc.com.
Meditrina, Inc. completes $10M equity financing led by Deerfield
SAN JOSE, Calif., June 1, 2020 /PRNewswire/ -- Meditrina, Inc., a premier women's healthcare company and developer of the state-of-the-art Aveta™ System, for the treatment of intrauterine pathologies such as polyps, fibroids, and/or RPOC, announced today that it has completed a $10 million equity financing as a first tranche of a $20- million commercialization round. The financing was led by Deerfield Management Company. Steven Hochberg, a Partner at Deerfield, will join the Meditrina Board of Directors.
"We are very excited about the potential of the Aveta System which allows for full control of procedures by integrating resection devices with a fluid management system, providing more accuracy and procedural efficiency in comparison to the alternative approaches," said Mr. Hochberg.
Meditrina's Aveta™ System was developed by entrepreneurs with a history of developing leading products in various healthcare fields, including, but not limited to, the EnSeal® tissue sealing devices, the Symphion® bi-polar tissue resection device, and the Minerva endometrial ablation system.
"The Aveta System was designed with full flexibility and scalability to perform various procedures in any setting, whether in an operating room or in a physician's office," said Csaba Truckai, President and CEO of Meditrina. "For the first time, fully integrated electronic hysteroscopes and resection devices have been developed for single use, which is a key differentiator from other decades-old technologies on the market. Single-use systems will greatly reduce the chance of infectious disease cross-contamination that can arise from re-usable hysteroscopes. We believe our single-use systems will provide significant clinical benefits, as well as lower the costs of diagnostic and therapeutic procedures," added Mr. Truckai.
"The Aveta System distances itself over the competition given the direct operator control of distending fluid flow and pressure in the operative field, along with a camera system that continually self-corrects the orientation of the surgical field. The resection blade is second to none in its efficiency and speed of excising difficult to remove myomas," said Dr. Steve Balaloski, OBGYN at WomanKind Obstetrics and Gynecology in Columbus, Ohio.
"Once you experience the Aveta System in a hysteroscopic surgery, it is hard to go back to using the old systems. It is a truly innovative product that is far more effective and efficient than the other competing products which I have used for years. My staff and I find so much less frustration with set-up and implementation as well," said Dr. Elizabeth Coronado, OBGYN Women's Specialists of Plano, Texas.
Abnormal uterine bleeding (AUB) is a common gynecologic problem, estimated to affect up to 30% of women.¹ Endometrial polyps are common, affecting 7.8%–41% of women occurring in premenopausal and postmenopausal women,² and approximately 25% to 50% of women with fibroids are symptomatic.³
Meditrina will use the proceeds from the financing to expand U.S. commercialization of the Aveta™ System and to further develop its product portfolio.
About Deerfield Deerfield is a healthcare investment management firm committed to advancing healthcare through investment, information and philanthropy.
About Meditrina, Inc. Founded in 2016, Meditrina, Inc. designs and develops innovative medical devices for minimally invasive gynecology. For more information, visit www.avetasystem.com.
Contact:Abbey Taylor
Director, Clinical Marketing
abbeyt@meditrina-inc.com
SOURCE Meditrina, Inc.
1 Arnold, Amy & Ketheeswaran, Ashradha & Bhatti, Mominah & Nesbitt-Hawes, Erin & Abbott, Jason. (2016). A Prospective Analysis of Hysteroscopic Morcellation in the Management of Intrauterine Pathologies. Journal of Minimally Invasive Gynecology. 23. 10.1016/j.jmig.2016.01.013.
2 Lee, S.C., Seibel, B. & Kaunitz, A.M. When Should Endometrial Polyps Be Treated?. Curr Obstet Gynecol Rep 1, 89–93 (2012).
3 Marsh, E. E., Al-Hendy, A., Kappus, D., Galitsky, A., Stewart, E. A., & Kerolous, M. (2018). Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. Journal of women's health (2002), 27(11), 1359–1367.
Addressing the COVID-19 Pandemic and the Impact on Hysteroscopic Procedures with the Aveta System
Keeping aligned with the recommendations for hysteroscopic procedures during and after the COVID-19 pandemic, stated by the Global Congress of Hysteroscopy Scientific Committee, is a priority to Meditrina Inc. Being able to treat all patients experiencing symptoms of abnormal uterine bleeding due to benign intrauterine pathologies is important during these challenging times.
The Aveta™ System, is a new mechanical hysteroscopy system intended for the removal and/or retrieval of intrauterine tissue such as, endometrial polyps, intrauterine fibroids, and/or RPOC, and provides “sterilization free” diagnostic and operative hysteroscopy because all devices are sterile and single use, minimizing set-up time, personnel requirements, and risks associated with cross contamination.
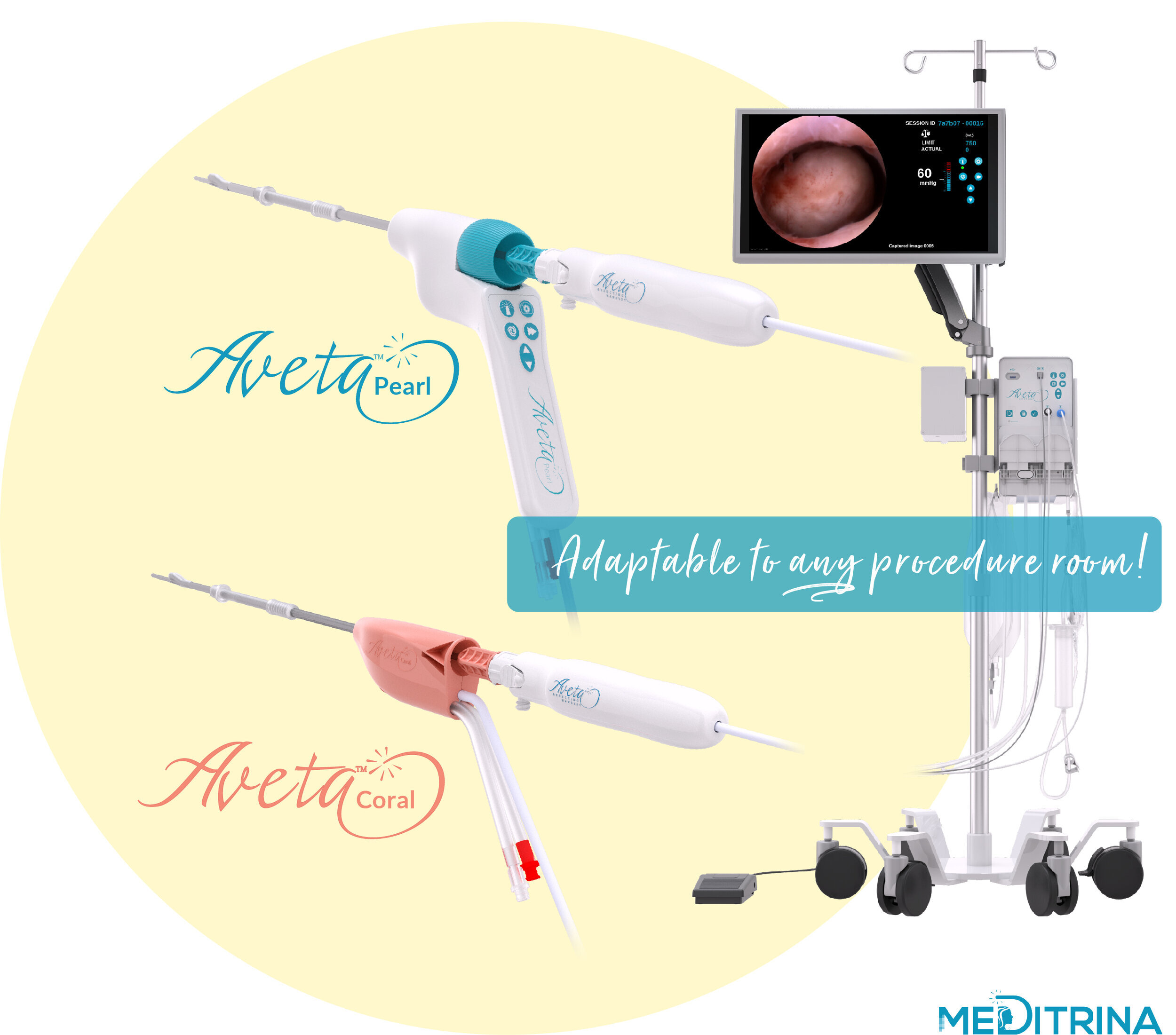
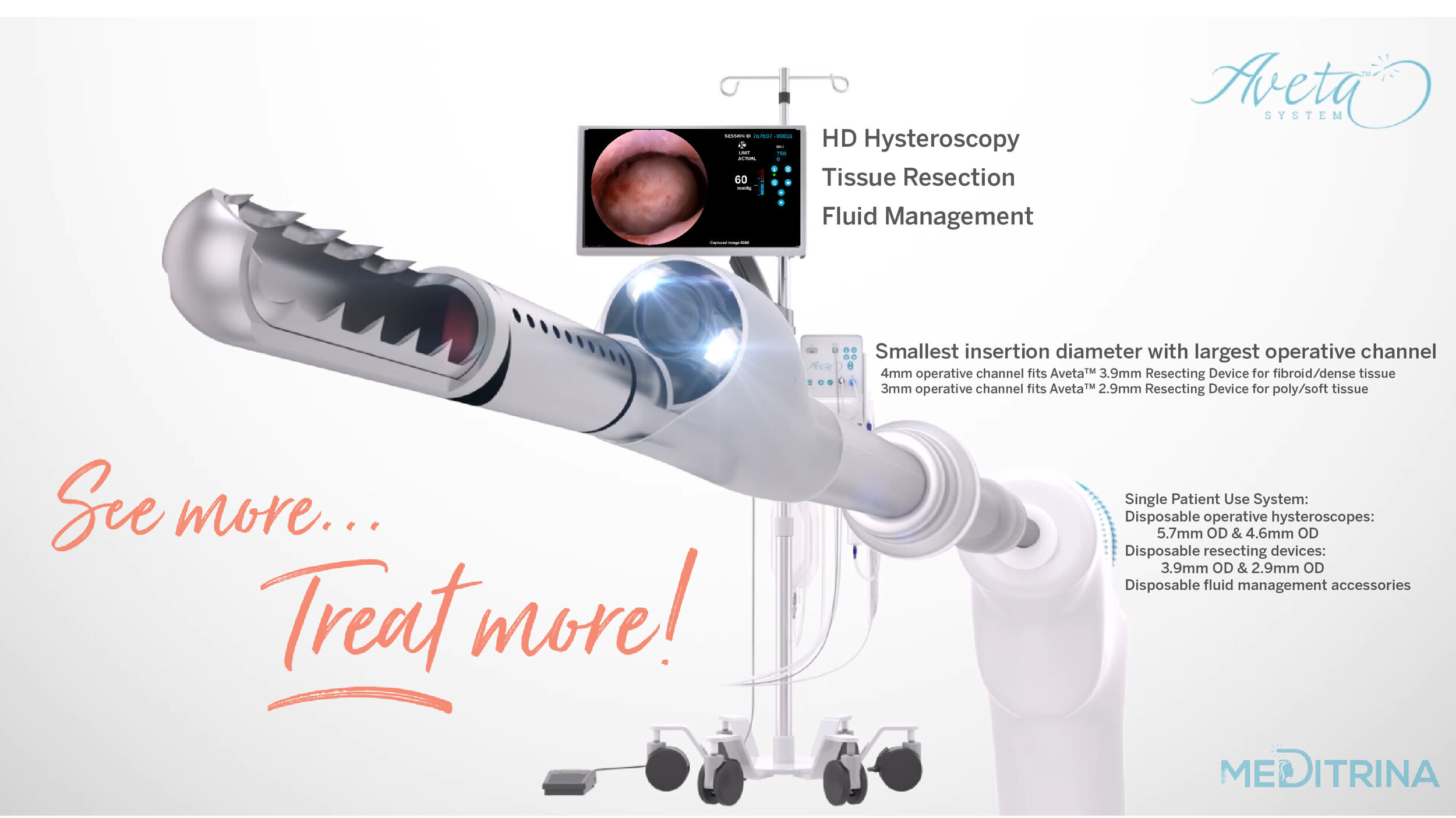
Our unique system can be used in any procedure room whether it’s in the hospital (no in-patient bed, operating suite, video tower, boom, etc. is necessary), ASC, or in-office, our system is easily adaptable to any room. We provide HD Video, superior fluid waste management, and tissue resection all controlled by one compact control box which is mounted onto a single rolling, adjustable saline pole with locking wheels.
We have two sizes of sterile single use Hysteroscopes and Resecting Devices available, dependent on patient comfort and tissue type. Our Hysteroscopes provide the largest working channel with the smallest insertion diameter, 4.6mm OD (3mm ID) and 5.7mm OD (4mm ID), respectively.
Aveta can also be used as a stand-alone fluid management system when used with any standard diagnostic or operative hysteroscope. Additionally, our proprietary sterile single use Hysteroscope can be used with a hanging bag for fluid management. The Aveta System is truly versatile and adaptable to any procedure room, and the surgeon control allows minimal staff when limited resources and PPE are not available.
One of the most overlooked steps during the process of decontaminating and reprocessing of reusable hysteroscopes is pre-cleaning. The steps for cleaning, high-level disinfection (HLD), rinsing, drying and storage for reusable hysteroscopes starts in the procedure room immediately post-op, not in the sterile processing department (SPD), due to having to remove patient biomaterials before biofilm hardening occurs. Proper training is involved to follow the manufacturer’s instructions for use and often takes multiple pages and hours just for the soaking portion of the process. When your staff is limited and/or new, there are a shortage of scopes, and patient turnaround is the highest importance, these steps for scope reprocessing can be burdensome, but are still necessary and required per the manufacturer’s IFU. Even missing one step can render the entire process ineffective and increase the risk of infection transmission. The Aveta System offers you and your patient the comfort of knowing that after each use devices can be easily discarded, eliminating the risk of device-related cross contamination.
In-Office Hysteroscopy Recommendations
Pre-procedure:
• The Aveta System is mechanical which means it does not produce surgical smoke during the case
• Our system contains HD Hysteroscopy video and monitor, fluid waste management and tissue resection on one rolling saline pole
• Our system has integrated inflow and suction outflow for tissue and fluid waste management, no external devices needed to perform hysteroscopy procedures
• Only one/two resources needed in the procedure room since Physician has full control of procedure, can be done with one person, if necessary, during COVID-19 pandemic (less PPE needed)
Intra-procedure:
• Can be done under local/regional anesthesia or conscious sedation due to smaller insertion diameter hysteroscopes available (patient dependent)
• The Aveta System has 5x* faster resection than currently marketed tissue removal devices making it a highly effective option
Post-procedure:
• Rapid procedure turnover because we provide multiple sizes of sterile single use devices that can be easily discarded immediately post-op which means less time needed for room decontamination. Patient can remain in same room with comfort and ease.
• No pre-cleaning, high level disinfection, rinsing, drying, storage required because we provide sterile single use hysteroscopes and resecting devices
Operating Room Hysteroscopy Recommendations
Pre-procedure:
• Aveta System is adaptable to any procedure room in the Hospital or ASC – no boom, surgical monitor, video towers, reusable scopes, light post adaptors or light sources needed to carry out procedures.
• Our system contains HD video, surgical monitor, fluid waste management and tissue resection on one rolling adjustable saline pole
• Only one/two resources needed in the operating room since Physician has full control of procedure, can be done with one person, if necessary, during COVID-19 pandemic (less PPE needed). Can be done on outpatient basis.
Intra-procedure:
• Can be done under local/regional anesthesia or conscious sedation due to smaller insertion diameter hysteroscopes available (patient dependent)
• The Aveta System has 5x* faster resection than currently marketed tissue removal devices making it a highly effective option
• The Aveta System is mechanical which means it does not produce surgical smoke during the case
• Our system has integrated inflow and suction outflow for tissue and fluid waste management, no external devices needed to perform hysteroscopy procedures
Post-procedure:
• Rapid operating room turnover because we provide multiple sizes of sterile single use devices that can be easily discarded immediately post-op which means less time needed for room decontamination.
• No pre-cleaning, high level disinfection, rinsing, drying, storage required because we provide sterile single use hysteroscopes and resecting devices
During the COVID-19 pandemic, patients are being limited to those whom delaying the procedure could result in adverse clinical outcomes. Why limit to only these patients when there are still patients who are seeking relief from their symptoms immediately? You can treat all patients during this challenging time (and after) because the Aveta System can adapt to any procedure room, not taking up inpatient beds, and can be done on an outpatient, same-day discharge. Most diagnostic and operative hysteroscopy procedures can be done under local/regional anesthesia or conscious sedation using our small insertion diameter Aveta Resecting Hysteroscopes.
Set up a virtual demonstration or email us at info@meditrina-inc.com for more information. We look forward to helping you during this time to treat all your patients, in all settings.
*Comparison of Tissue Resection Rates of The Myosure, Truclear, And Aveta Hysteroscopic Tissue Removal Systems. A Three-Arm, Bench Study. A.I. Brill
©2020 Meditrina, Inc. or its affiliates. Aveta™ is a registered trademark of Meditrina, Inc. All rights reserved.
Solutions and Alternatives to Hospital-Based Care for GYN Surgical Patients with the Aveta™ Sytem
As we move closer toward the return of elective procedures, the Aveta™ System is uniquely positioned to meet the changing needs of patients and surgeons for hysteroscopic procedures with the flexibility to migrate those cases from the hospital to the ASC or office setting. Aligned with key patient safety initiatives, Aveta™ provides the only single-use resecting hysteroscope designed to reduce the risks and delays associated with ineffective reprocessing. With an all-in-one versatile system (HD vision, enhanced resection, optimized fluid management), we have solved the frustration and inefficiency with hysteroscopy setup/breakdown by enabling rapid OR/Clinic turnover with procedure room adaptability. To schedule a virtual discussion or demonstration please contact us at info@meditrina-inc.com.
Introducing the Aveta™ System - a new “See and Treat” Hysteroscopy solution
Introducing the Aveta™ System, a new “See and Treat” Hysteroscopy solution featuring a Single-Use Resecting Hysteroscope with HD Vision and the smallest insertion diameter with the largest operative channel. Hysteroscopy meets innovation with 5X faster tissue resection for uterine polyps and fibroids.