Premiere Aveta Webinar | Meditrina Presents: The Good, the Bad, and the Ugly of Hysteroscopic Systems
Join three experienced hysteroscopists, Dr. Peter Heinlein, Dr. Zoyla Almeida, and Dr. Erica Stockwell, as they share comparative insights from extensive real-world use of leading hysteroscopic systems such as, Aveta, MyoSure, TruClear, Symphion, and traditional resectoscopes.
Moderated by Dr. Peter Heinlein.
Through discussion of case experiences and procedural nuances, these three expert speakers will highlight differences in visualization, tissue removal efficiency, ergonomics, and patient comfort. Attendees will gain a deeper understanding of system selection and optimization for all procedural settings. This session is designed for gynecologists and women’s health professionals seeking practical, experience-based perspectives on current hysteroscopic technologies.
Myomectomy using the Aveta System is shown and narrated by Dr. Erica Stockwell, Advent Health FL.
Meditrina announces strategic partnership with M.E.D. Surgical to distribute Aveta® System in Ireland and UK
M.E.D Surgical, a division of Uniphar Medtech, today announced a strategic partnership with Meditrina to distribute the innovative Aveta® System throughout Ireland and the United Kingdom. This collaboration combines M.E.D. Surgical's extensive distribution network with Meditrina's groundbreaking women’s health technology.
The Aveta® System is a state-of-the-art medical device designed to provide minimally invasive treatment options for women experiencing abnormal uterine bleeding, fibroids, and other intrauterine pathologies. The Aveta® System was developed for hysteroscopy procedures, allowing healthcare providers to directly visualize and treat intrauterine conditions with precision and minimal patient discomfort.
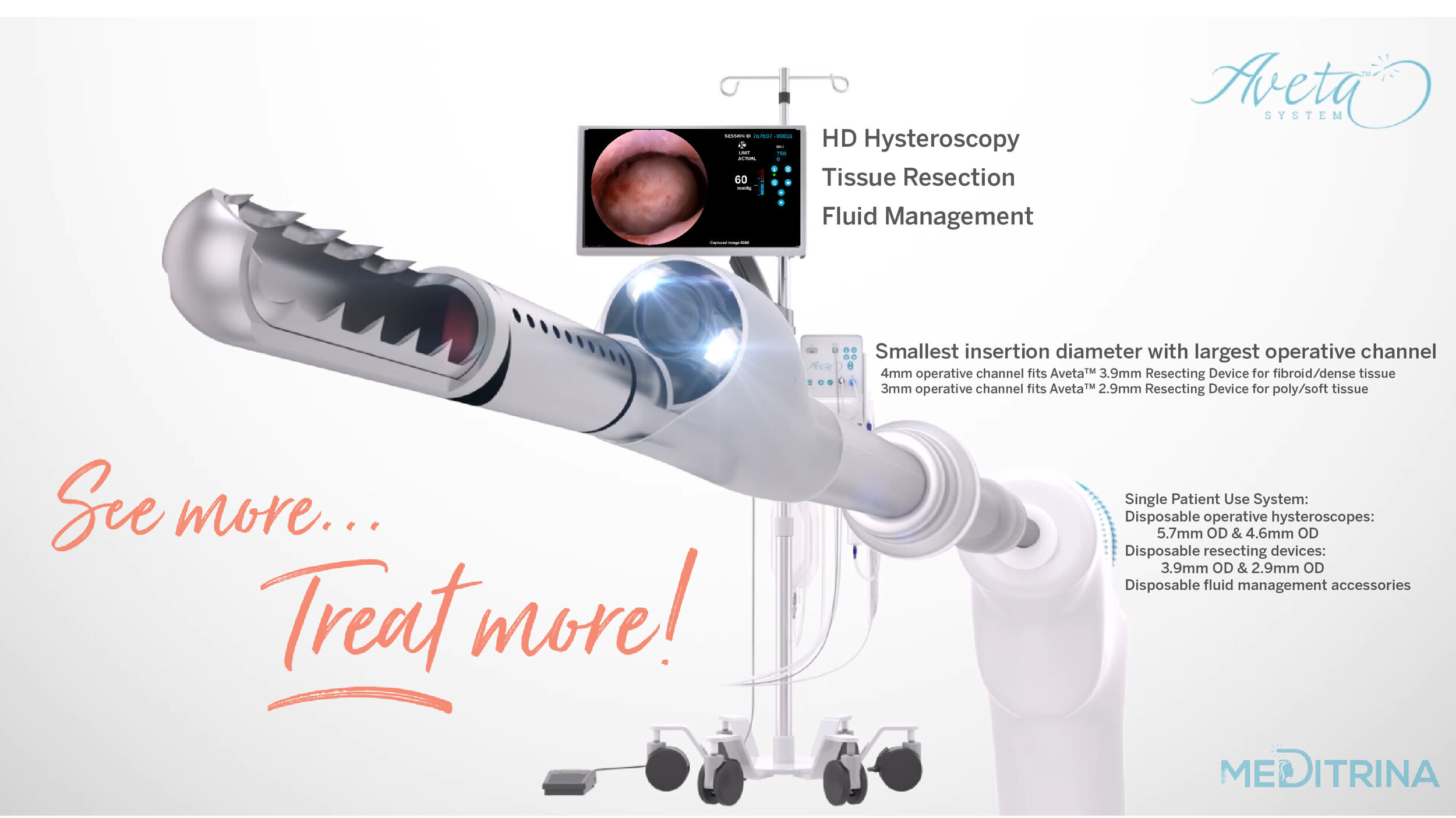
This FDA-cleared, CE-marked, and UKCA-marked technology offers healthcare providers a safe and effective solution for patients seeking alternatives to traditional surgical interventions. By leveraging advanced hysteroscopic techniques, the Aveta® System, features the smallest insertion diameter with largest working channel operative hysteroscope on the market, enabling detailed examination and targeted treatment of various gynaecological conditions, reducing the need for more invasive surgical procedures.
"We are thrilled to partner with Meditrina to bring the Aveta® System to healthcare providers across Ireland and the UK," said David Laheen, Managing Director of M.E.D. Surgical. "This partnership aligns perfectly with our commitment to delivering innovative medical technologies that improve patient outcomes while reducing healthcare costs."
The Aveta® System's proprietary technology allows gynaecologists to perform procedures safe and effectively in any procedure setting, potentially reducing recovery time, and eliminating the need for general anaesthesia in many cases. Clinical evaluations have demonstrated excellent safety profiles and high user satisfaction rates.
"Meditrina is proud to collaborate with M.E.D. Surgical to expand access to the Aveta® System," said Csaba Truckai, CEO of Meditrina. "Their extensive experience in the medical device sector and strong relationships with healthcare providers make them the ideal partner to help us reach more patients who can benefit from our technology."
The partnership addresses the growing healthcare industry focus on cost-effective treatments that minimize hospital stays while potentially improving patient quality of life.
M.E.D. Surgical will begin distributing the Aveta® System immediately, with comprehensive training and support programs available to healthcare providers interested in adopting this technology.
About M.E.D Surgical
M.E.D Surgical, a division of Uniphar Medtech, is a leading distributor of innovative medical technologies throughout Ireland and the United Kingdom. With decades of experience in the healthcare industry, M.E.D. Surgical partners with leading medtech manufacturers worldwide to bring cutting-edge medical solutions to healthcare providers, focusing on improving patient outcomes and healthcare delivery.
About Meditrina
Meditrina is a medical technology company dedicated to advancing women's health through innovative, minimally invasive solutions. The company's flagship product, the Aveta® System, represents a significant advancement in treating intrauterine pathologies, offering patients and providers an effective alternative to more invasive surgical procedures.
Meditrina Announces 510(k) Clearance for Gen 2 Bipolar RF Hysteroscopy System and Aveta Glo Bipolar RF Device
SAN JOSE, Calif., May 17, 2024 /PRNewswire/ -- Meditrina, a key leader in women's health medical devices, is thrilled to announce the FDA 510(k) clearance of its Gen 2 bipolar RF hysteroscopy system. This new system features bipolar radiofrequency technology, and a new bipolar RF device called the Aveta Glo, representing a significant advancement in minimally invasive gynecologic procedures.
The Aveta System 2.0 builds on the success of its first-generation system, introduced in 2019, allowing physicians full control of procedures from the hysteroscope handle with integrated resection and fluid management. The system is complimented by several pathology-optimized resection devices to treat soft to calcified intrauterine pathologies resulting in 10-minute¹ faster operating room turnover and significantly faster resection time improving the procedural and facility efficiency compared to existing modalities. The new System's bipolar radiofrequency technology allows for more controlled and efficient tissue removal with hemostasis, potentially improving patient outcomes. Additionally, the new bipolar RF device offers enhanced versatility, enabling physicians to perform a wider range of diagnostic and therapeutic procedures including the ability to address fibroids with deeper myometrial wall penetration across any density tissue type and provide hemostasis, when needed, expanding Meditrina's market opportunity an additional $1.5B.
"The clearance of our Gen 2 system marks a significant milestone for Meditrina and underscores our commitment to advancing women's health through innovative medical technologies," said Csaba Truckai, CEO of Meditrina. "We are dedicated to providing healthcare professionals with the tools they need to deliver the highest standard of care, and our new system exemplifies this mission by offering unparalleled performance."
Meditrina remains committed to providing comprehensive training and support for healthcare professionals to ensure the seamless integration of the Aveta System into clinical practices. This commitment reflects the company's dedication to empowering medical professionals with the tools they need to deliver optimal patient care.
In tandem with this regulatory achievement, Meditrina is also excited to announce the completion of the last $5Mtranche of the $77M series C investment led by Deerfield Management Company, ShangBay Capital and other insiders. The investment is combined with an additional $5 million debt financing by SLR Capital Partners. The company is already approaching to be cash flow positive, and the additional funds will accelerate new product introduction and expansion of the sales force. This successful financing effort underscores strong investor confidence in the company's vision and growth trajectory.
"We are immensely grateful for the continued support of our investors," said Csaba Truckai, CEO of Meditrina. "This financing not only validates our strategic direction but also provides the necessary resources to scale our operations and bring our innovative solutions to a global market. We are poised for substantial growth and are confident in our ability to achieve cash flow positivity soon."
Meditrina will continue striving to better women's health, with a focus on delivering cutting-edge medical devices that enhance the quality of care for patients worldwide. Aveta System 2.0 is set to redefine standards in the field, providing healthcare professionals with advanced tools to improve outcomes and patient experiences.
About Meditrina:
Founded in 2016, Meditrina, Inc. designs and develops innovative medical devices for minimally invasive gynecology. For more information, visit www.avetasystem.com.
¹Data on file at Meditrina inc.; survey conducted Oct 2019-Aug 2020 of n56 OB/GYN users and n38 Nurse users.Meditrina Announces CE Mark Approval and First Multi-International Cases Performed
SAN JOSE, Calif., May 13, 2024 /PRNewswire/ -- Meditrina, a leading innovator in gynecologic medical devices, announces the successful receipt of UKCA Mark, and CE Mark approval in accordance with Regulation (EU) 2017/745, for its state-of-the-art Aveta Hysteroscopy System. With this significant milestone achieved, Meditrina is thrilled to enter international markets, marking a pivotal moment in the company's commitment to advancing women's health globally.
Receiving the UKCA and CE Mark approval underscores Meditrina's dedication to meeting the highest standards of quality, safety, and efficacy required for market entry in the European Union and the UK. This achievement not only validates the company's commitment to regulatory compliance but also positions Meditrina as a trusted provider of cutting-edge medical solutions.
Hysteroscopy is a crucial diagnostic and therapeutic procedure used to evaluate and treat conditions affecting the uterus. Meditrina's Aveta hysteroscopy system combines advanced technology with ergonomic design, enabling healthcare professionals to perform minimally invasive procedures with enhanced visualization and control, ultimately leading to improved patient outcomes and experiences.
"We are excited to receive UKCA and CE Mark approval for our hysteroscopy system, as it validates the exceptional quality and performance of our product," remarked Csaba Truckai, CEO of Meditrina. "Furthermore, the successful completion of our initial international procedures in Spain and Denmark signifies a significant milestone in our mission to revolutionize women's healthcare on a global scale. We are proud to collaborate with healthcare providers worldwide in delivering advanced solutions that empower women to lead healthier lives."
The appointment of Ray Gerena as the Senior Vice President of Global Sales further strengthens Meditrina's commitment to driving growth and expanding its global footprint. With Ray's extensive experience and leadership in strategic sales initiatives, Meditrina is well-positioned to capitalize on new market opportunities and forge strong partnerships with healthcare providers worldwide.
Ray remarked, "I am honored to join Meditrina at such an exciting time in its journey. The UKCA and CE Mark approval and successful international procedures represent significant milestones for the company, and I am eager to lead our global sales efforts in bringing our innovative hysteroscopy system to healthcare providers around the world. Together, we will continue to make a meaningful impact on women's health."
The inaugural international procedures conducted in Spain and Denmark exemplify the growing demand for Meditrina's groundbreaking hysteroscopy system beyond the United States. Healthcare professionals in Spain and Denmark have embraced the technology's capabilities, recognizing its potential in the diagnosis and treatment of various gynecological conditions while prioritizing patient comfort and safety.
Through ongoing innovation and collaboration, Meditrina remains steadfast in its commitment to empowering healthcare professionals and improving outcomes for women worldwide.
About Meditrina:
Founded in 2016, Meditrina, Inc. designs and develops innovative medical devices for minimally invasive gynecology. For more information, visit www.avetasystem.com.
Contact:
Abbey Taylor
Associate VP of Marketing
abbeyt@meditrina-inc.com
SOURCE Meditrina Inc.
Aveta® is a registered trademark of Meditrina Inc.
SOURCE Meditrina, Inc.
Find Out Why Byron Hapner, DO, and His Inspira Colleagues Switched to Aveta
Find out why Byron Hapner, DO, and his Inspira Medical Center Mullica Hill colleagues switched to Aveta for all their diagnostic and therapeutic hysteroscopies, including calcified myomectomies. Watch the video to learn about some of the many Aveta features that can benefit gynecologic surgeons. Schedule a trial today by contacting us at info@meditrina-inc.com.
Dr. Byron Hapner, DO, removes a calcified fibroid using the Aveta Hysteroscopy System.
Aveta | the System that puts You in Control
The Aveta System brings precise control to your hysteroscopy procedures. Its physician-controlled scope lets you take HD images, adjust pressure and fluid deficit, enable irrigation and on-demand rapid flush, and control fluid management, all from the handle. With a head-up display, all the important information you need is always right in front of you. With Aveta, you can focus on what’s most important, your patient, without having to look away to search for help if resources are limited.
Dr. Geoffrey Bowers Axia Women’s Health Resects 2cm Partially Calcified Fibroid | Aveta
Watch Dr. Geoffrey Bowers with Axia Women's Health South Jersey, resect a 2cm partially calcified myoma using the Aveta System. Schedule a trial today by contacting us at info@meditrina-inc.com.
2021 AAGL Meeting in Austin, TX | Aveta System
Thank you AAGL for hosting another great educational meeting for #MIGS and industry professionals to network and connect! Was a great opportunity to join the hands-on hysteroscopy lab, 614-HSC “Advancing Your Hysteroscopy Skills with Global Experts” co-chaired by Dr. Amy Garcia and Dr. Attilio Di Spiezio Sardo. Also, a big thanks to everyone who was able to stop by our booth for a personalized demo of the Aveta System!
Meditrina Expands Hysteroscopic Tissue Resection Product Portfolio with the Release of the New Aveta® Office Suite
SAN JOSE, Calif., July 9, 2021 /PRNewswire/ -- Meditrina Inc. announces the market release of their single-use Aveta Opal Hysteroscope and disposable Aveta Auto Resecting Device for removing soft endometrial tissue such as uterine polyps in an office setting, without the need for fluid management.
The Aveta Opal HD Hysteroscope has the smallest insertion profile at 4.6mm featuring a 3.0mm working channel optimized for use with the 2.9 mm OD Aveta Auto Resecting Device, which are now part of the newly introduced Aveta office suite product portfolio. The Aveta Opal and Aveta Coral are the only commercially available hysteroscopes that have the capability to resect both fibroids and polyps without the need for a 5.5mm sheath and increased dilatation, ideal for post-menopausal patients and those with a stenotic cervix.
The Aveta Auto sets itself apart from existing devices on the market due to its internal handle mechanics. Situated inside the handle is a motorized internal fluid management suction pump and resection drive. Additionally, there is an integrated tissue specimen container attached to the device for convenient pathology collection. The automated tissue removal is faster than anything else offered commercially and eliminates fatigue due to manual hand squeezing for tissue resection. Dissatisfaction with manual devices has resulted in costly blades being utilized to complete cases, therefore, decreasing profitability.
"OBGYN's have long been searching for a platform that allows them to fully integrate "see and treat" methodology since the 58558 code was revalued by CMS. "See and treat" has proven cost savings to both patient and buyer. Upfront investment and inability to treat complex pathology have been the two biggest barriers to adoption.
Gynecologists acquiring the Aveta office suite can address a full range of pathology with unparalleled performance and cost effectiveness. The Aveta Opal Single-Use Hysteroscope is fully scalable and can quickly be converted from a pressurized saline bag to a full fluid management procedure when complex pathology is detected. The Aveta Opal Scope gives Gynecologists an economic and safe alternative for performing hysteroscopies in the office with full O.R. performance." – Matt Fratantoro, Meditrina VP of Sales and Marketing.
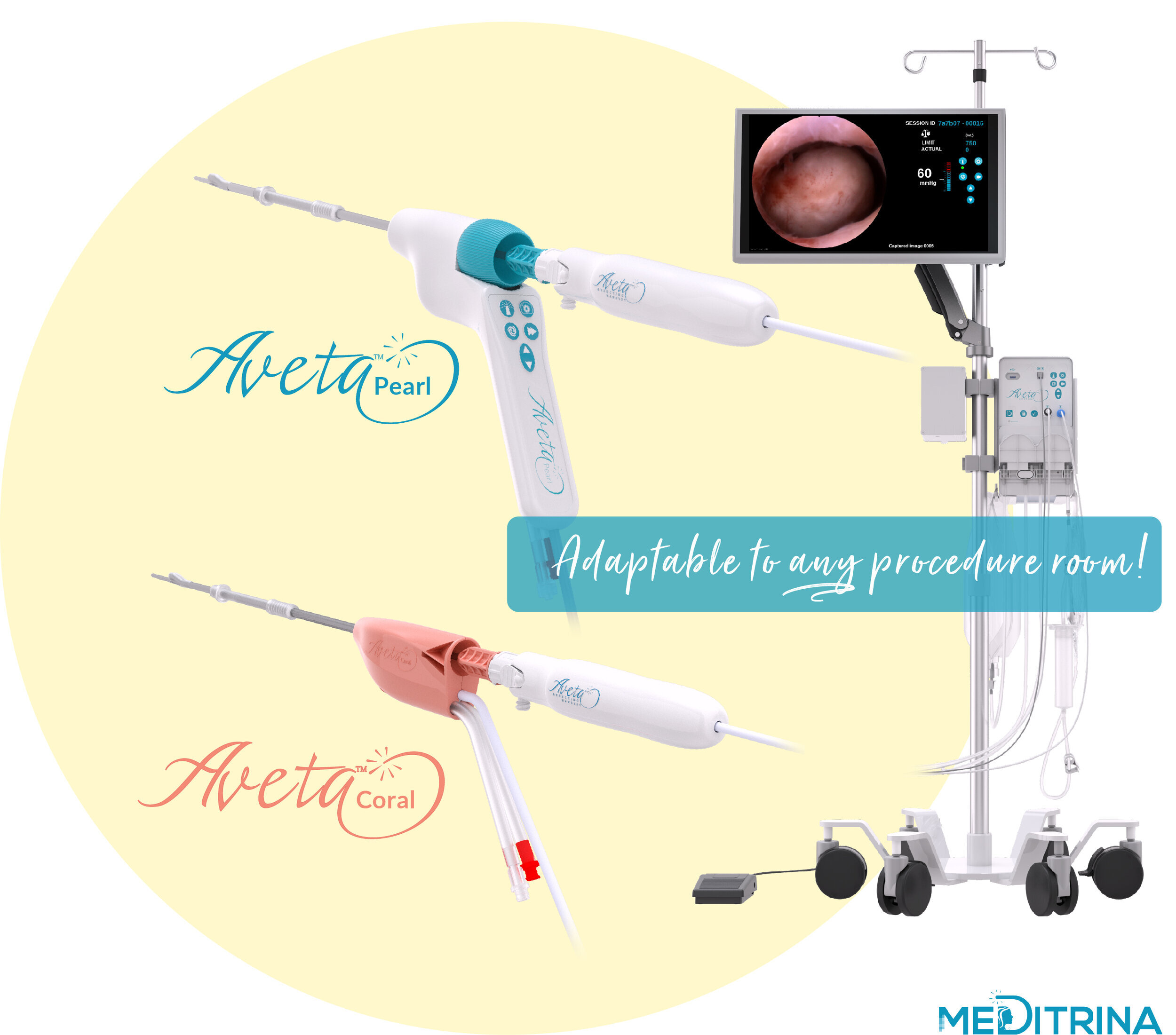
The new Aveta devices compliment an already existing suite of innovative women's health products which include, Aveta Coral 4.6mm and Aveta Pearl 5.7mm Single-Use Hysteroscopes, Aveta Smol 2.9mm, Aveta Flex 2.9mm, and Aveta Max 3.9mm single-use resecting devices.
Contact:
Abbey Taylor
Director, Clinical Marketing
abbeyt@meditrina-inc.com
SOURCE Meditrina, Inc.
Meditrina, Inc. completes $10M equity financing led by Deerfield
SAN JOSE, Calif., June 1, 2020 /PRNewswire/ -- Meditrina, Inc., a premier women's healthcare company and developer of the state-of-the-art Aveta™ System, for the treatment of intrauterine pathologies such as polyps, fibroids, and/or RPOC, announced today that it has completed a $10 million equity financing as a first tranche of a $20- million commercialization round. The financing was led by Deerfield Management Company. Steven Hochberg, a Partner at Deerfield, will join the Meditrina Board of Directors.
"We are very excited about the potential of the Aveta System which allows for full control of procedures by integrating resection devices with a fluid management system, providing more accuracy and procedural efficiency in comparison to the alternative approaches," said Mr. Hochberg.
Meditrina's Aveta™ System was developed by entrepreneurs with a history of developing leading products in various healthcare fields, including, but not limited to, the EnSeal® tissue sealing devices, the Symphion® bi-polar tissue resection device, and the Minerva endometrial ablation system.
"The Aveta System was designed with full flexibility and scalability to perform various procedures in any setting, whether in an operating room or in a physician's office," said Csaba Truckai, President and CEO of Meditrina. "For the first time, fully integrated electronic hysteroscopes and resection devices have been developed for single use, which is a key differentiator from other decades-old technologies on the market. Single-use systems will greatly reduce the chance of infectious disease cross-contamination that can arise from re-usable hysteroscopes. We believe our single-use systems will provide significant clinical benefits, as well as lower the costs of diagnostic and therapeutic procedures," added Mr. Truckai.
"The Aveta System distances itself over the competition given the direct operator control of distending fluid flow and pressure in the operative field, along with a camera system that continually self-corrects the orientation of the surgical field. The resection blade is second to none in its efficiency and speed of excising difficult to remove myomas," said Dr. Steve Balaloski, OBGYN at WomanKind Obstetrics and Gynecology in Columbus, Ohio.
"Once you experience the Aveta System in a hysteroscopic surgery, it is hard to go back to using the old systems. It is a truly innovative product that is far more effective and efficient than the other competing products which I have used for years. My staff and I find so much less frustration with set-up and implementation as well," said Dr. Elizabeth Coronado, OBGYN Women's Specialists of Plano, Texas.
Abnormal uterine bleeding (AUB) is a common gynecologic problem, estimated to affect up to 30% of women.¹ Endometrial polyps are common, affecting 7.8%–41% of women occurring in premenopausal and postmenopausal women,² and approximately 25% to 50% of women with fibroids are symptomatic.³
Meditrina will use the proceeds from the financing to expand U.S. commercialization of the Aveta™ System and to further develop its product portfolio.
About Deerfield Deerfield is a healthcare investment management firm committed to advancing healthcare through investment, information and philanthropy.
About Meditrina, Inc. Founded in 2016, Meditrina, Inc. designs and develops innovative medical devices for minimally invasive gynecology. For more information, visit www.avetasystem.com.
Contact:Abbey Taylor
Director, Clinical Marketing
abbeyt@meditrina-inc.com
SOURCE Meditrina, Inc.
1 Arnold, Amy & Ketheeswaran, Ashradha & Bhatti, Mominah & Nesbitt-Hawes, Erin & Abbott, Jason. (2016). A Prospective Analysis of Hysteroscopic Morcellation in the Management of Intrauterine Pathologies. Journal of Minimally Invasive Gynecology. 23. 10.1016/j.jmig.2016.01.013.
2 Lee, S.C., Seibel, B. & Kaunitz, A.M. When Should Endometrial Polyps Be Treated?. Curr Obstet Gynecol Rep 1, 89–93 (2012).
3 Marsh, E. E., Al-Hendy, A., Kappus, D., Galitsky, A., Stewart, E. A., & Kerolous, M. (2018). Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. Journal of women's health (2002), 27(11), 1359–1367.
Addressing the COVID-19 Pandemic and the Impact on Hysteroscopic Procedures with the Aveta System
Keeping aligned with the recommendations for hysteroscopic procedures during and after the COVID-19 pandemic, stated by the Global Congress of Hysteroscopy Scientific Committee, is a priority to Meditrina Inc. Being able to treat all patients experiencing symptoms of abnormal uterine bleeding due to benign intrauterine pathologies is important during these challenging times.
The Aveta™ System, is a new mechanical hysteroscopy system intended for the removal and/or retrieval of intrauterine tissue such as, endometrial polyps, intrauterine fibroids, and/or RPOC, and provides “sterilization free” diagnostic and operative hysteroscopy because all devices are sterile and single use, minimizing set-up time, personnel requirements, and risks associated with cross contamination.
Our unique system can be used in any procedure room whether it’s in the hospital (no in-patient bed, operating suite, video tower, boom, etc. is necessary), ASC, or in-office, our system is easily adaptable to any room. We provide HD Video, superior fluid waste management, and tissue resection all controlled by one compact control box which is mounted onto a single rolling, adjustable saline pole with locking wheels.
We have two sizes of sterile single use Hysteroscopes and Resecting Devices available, dependent on patient comfort and tissue type. Our Hysteroscopes provide the largest working channel with the smallest insertion diameter, 4.6mm OD (3mm ID) and 5.7mm OD (4mm ID), respectively.
Aveta can also be used as a stand-alone fluid management system when used with any standard diagnostic or operative hysteroscope. Additionally, our proprietary sterile single use Hysteroscope can be used with a hanging bag for fluid management. The Aveta System is truly versatile and adaptable to any procedure room, and the surgeon control allows minimal staff when limited resources and PPE are not available.
One of the most overlooked steps during the process of decontaminating and reprocessing of reusable hysteroscopes is pre-cleaning. The steps for cleaning, high-level disinfection (HLD), rinsing, drying and storage for reusable hysteroscopes starts in the procedure room immediately post-op, not in the sterile processing department (SPD), due to having to remove patient biomaterials before biofilm hardening occurs. Proper training is involved to follow the manufacturer’s instructions for use and often takes multiple pages and hours just for the soaking portion of the process. When your staff is limited and/or new, there are a shortage of scopes, and patient turnaround is the highest importance, these steps for scope reprocessing can be burdensome, but are still necessary and required per the manufacturer’s IFU. Even missing one step can render the entire process ineffective and increase the risk of infection transmission. The Aveta System offers you and your patient the comfort of knowing that after each use devices can be easily discarded, eliminating the risk of device-related cross contamination.
In-Office Hysteroscopy Recommendations
Pre-procedure:
• The Aveta System is mechanical which means it does not produce surgical smoke during the case
• Our system contains HD Hysteroscopy video and monitor, fluid waste management and tissue resection on one rolling saline pole
• Our system has integrated inflow and suction outflow for tissue and fluid waste management, no external devices needed to perform hysteroscopy procedures
• Only one/two resources needed in the procedure room since Physician has full control of procedure, can be done with one person, if necessary, during COVID-19 pandemic (less PPE needed)
Intra-procedure:
• Can be done under local/regional anesthesia or conscious sedation due to smaller insertion diameter hysteroscopes available (patient dependent)
• The Aveta System has 5x* faster resection than currently marketed tissue removal devices making it a highly effective option
Post-procedure:
• Rapid procedure turnover because we provide multiple sizes of sterile single use devices that can be easily discarded immediately post-op which means less time needed for room decontamination. Patient can remain in same room with comfort and ease.
• No pre-cleaning, high level disinfection, rinsing, drying, storage required because we provide sterile single use hysteroscopes and resecting devices
Operating Room Hysteroscopy Recommendations
Pre-procedure:
• Aveta System is adaptable to any procedure room in the Hospital or ASC – no boom, surgical monitor, video towers, reusable scopes, light post adaptors or light sources needed to carry out procedures.
• Our system contains HD video, surgical monitor, fluid waste management and tissue resection on one rolling adjustable saline pole
• Only one/two resources needed in the operating room since Physician has full control of procedure, can be done with one person, if necessary, during COVID-19 pandemic (less PPE needed). Can be done on outpatient basis.
Intra-procedure:
• Can be done under local/regional anesthesia or conscious sedation due to smaller insertion diameter hysteroscopes available (patient dependent)
• The Aveta System has 5x* faster resection than currently marketed tissue removal devices making it a highly effective option
• The Aveta System is mechanical which means it does not produce surgical smoke during the case
• Our system has integrated inflow and suction outflow for tissue and fluid waste management, no external devices needed to perform hysteroscopy procedures
Post-procedure:
• Rapid operating room turnover because we provide multiple sizes of sterile single use devices that can be easily discarded immediately post-op which means less time needed for room decontamination.
• No pre-cleaning, high level disinfection, rinsing, drying, storage required because we provide sterile single use hysteroscopes and resecting devices
During the COVID-19 pandemic, patients are being limited to those whom delaying the procedure could result in adverse clinical outcomes. Why limit to only these patients when there are still patients who are seeking relief from their symptoms immediately? You can treat all patients during this challenging time (and after) because the Aveta System can adapt to any procedure room, not taking up inpatient beds, and can be done on an outpatient, same-day discharge. Most diagnostic and operative hysteroscopy procedures can be done under local/regional anesthesia or conscious sedation using our small insertion diameter Aveta Resecting Hysteroscopes.
Set up a virtual demonstration or email us at info@meditrina-inc.com for more information. We look forward to helping you during this time to treat all your patients, in all settings.
*Comparison of Tissue Resection Rates of The Myosure, Truclear, And Aveta Hysteroscopic Tissue Removal Systems. A Three-Arm, Bench Study. A.I. Brill
©2020 Meditrina, Inc. or its affiliates. Aveta™ is a registered trademark of Meditrina, Inc. All rights reserved.
Solutions and Alternatives to Hospital-Based Care for GYN Surgical Patients with the Aveta™ Sytem
As we move closer toward the return of elective procedures, the Aveta™ System is uniquely positioned to meet the changing needs of patients and surgeons for hysteroscopic procedures with the flexibility to migrate those cases from the hospital to the ASC or office setting. Aligned with key patient safety initiatives, Aveta™ provides the only single-use resecting hysteroscope designed to reduce the risks and delays associated with ineffective reprocessing. With an all-in-one versatile system (HD vision, enhanced resection, optimized fluid management), we have solved the frustration and inefficiency with hysteroscopy setup/breakdown by enabling rapid OR/Clinic turnover with procedure room adaptability. To schedule a virtual discussion or demonstration please contact us at info@meditrina-inc.com.
Introducing the Aveta™ System - a new “See and Treat” Hysteroscopy solution
Introducing the Aveta™ System, a new “See and Treat” Hysteroscopy solution featuring a Single-Use Resecting Hysteroscope with HD Vision and the smallest insertion diameter with the largest operative channel. Hysteroscopy meets innovation with 5X faster tissue resection for uterine polyps and fibroids.
Meditrina to Introduce Aveta™ System for Diagnostic and Therapeutic Hysteroscopy at 2019 AAGL
SAN JOSE, Calif., Nov. 8, 2019 /PRNewswire/ -- Meditrina, Inc. will launch the Aveta™ Hysteroscopy System at the 2019 American Association of Gynecologic Laparoscopists meeting.
The Aveta™ System is a new hysteroscopy platform that combines HD video, fluid management and tissue resection into one seamless system
SAN JOSE, Calif., Nov. 8, 2019 /PRNewswire/ -- Meditrina, Inc. will launch the Aveta™ Hysteroscopy System at the 2019 American Association of Gynecologic Laparoscopists meeting.
The Aveta™ hysteroscopy suite was designed to provide physicians with the best care in their diagnosis and treatment of endometrial polyps and uterine fibroids. The system seamlessly integrates into any operating, procedure, or exam room. This new hysteroscopy system is an all-in-one platform which includes the single-use Aveta™ Pearl Hysteroscope for myomectomy, and the single-use Aveta™ Coral Hysteroscope for polypectomy procedures with integrated tissue resection and fluid management capability. The Aveta™ Pearl Hysteroscope is the first single-use hysteroscope to include full physician control providing proprietary fluid management and video control function directly on the hysteroscope handle.
Both HD wide field of view Aveta™ Hysteroscopes include patented expandable working channel technology providing the largest working channel with the smallest insertion diameter on the market. Additionally, both Hysteroscopes contain a proprietary image orientation lock feature which constantly keeps the image in an upright position, thus eliminating awkward tubing, light cable, and camera head management, while ensuring a consistent frame of reference and ease of use for the physician, with minimal patient discomfort. The simplified setup of the integrated fluid management was designed to facilitate the nursing staff in supporting procedures with ease and reduce OR setup time. The Aveta™ Fluid Management was also cleared by the FDA to be used as a standalone fluid management system with selected standard hysteroscopes.
The proprietary Aveta™ 3.9mm Resecting Device, is capable of removing over 20g/min of fibroid tissue,¹ and Monday, November 11th at 12:17pm, Dr. Andrew Brill, will present, Comparison of Tissue Resection Rates of The MyoSure, TruClear, and Aveta Hysteroscopic Tissue Removal Systems. A Three-Arm, Bench Study, during Open Communications 3 Hysteroscopy, at the 2019 AAGL Meeting.
About Meditrina, Inc.
Founded in 2016, Meditrina, Inc. is a small research and development engineering startup which designs and develops innovative medical devices for minimally invasive gynecology. For more information, visit www.avetasystem.com.
References
1. Comparison of Tissue Resection Rates of the Myosure, Truclear, and Aveta Hysteroscopic Tissue Removal Systems. A ThreeArm, Bench Study
Brill, AI
Journal of Minimally Invasive Gynecology, Volume 26, Issue 7, S29 - S30
Meditrina, Inc. completes $13 million equity financing led by ShangBay Capital LLC and Aethan Capital, Inc.
Meditrina, Inc., a women's healthcare company and the developer of the Aveta™ System for the treatment of various intrauterine pathologies such as polyps and fibroids, announced today that it has completed a $13 million equity financing. The financing was led by ShangBay Capital LLC and Aethan Capital, Inc.
SAN JOSE, Calif., Oct. 1, 2019 /PRNewswire/ -- Meditrina, Inc., a women's healthcare company and the developer of the Aveta™ System for the treatment of various intrauterine pathologies such as polyps and fibroids, announced today that it has completed a $13 million equity financing. The financing was led by ShangBay Capital LLC and Aethan Capital, Inc.
The Aveta™ System is intended for hysteroscopic tissue chip resection under continuous flow conditions using a proprietary expandable working channel hysteroscope, combined with a fluid management and resection system, where the physician is able to control all parameters of the procedure and view it on a single monitor. Concurrent with the financing, William Dai, Founding Managing Partner of ShangBay Capital, will join the Meditrina Board of Directors.
"We are very excited about the potential of the Aveta™ System to establish a new gold standard in hysteroscopic resection procedures. Meditrina has developed multiple proprietary technology platforms including but not limited to, an expandable working channel hysteroscope, a high-speed mechanical oscillation mechanism, fluid control algorithm, and an HD CMOS visualization and fluid management system," said Mr. Dai.
Meditrina will use the proceeds from the financing to launch the US commercialization of the Aveta™ System that received U.S. Food and Drug Administration (FDA) 510(k) clearance in May 2019.
"On behalf of all of the shareholders in Meditrina, Inc., I welcome ShangBay Capital as a new investor and William as a new member of our Board," said Csaba Truckai, President and CEO of Meditrina, Inc.
According to the New England Journal of Medicine, approximately 1,000,000 women suffer from intrauterine polyp and fibroid related issues that requires surgical treatment.
About ShangBay Capital LLC
ShangBay Capital is a venture capital company that brings investors access to best-in-class investments in U.S. medical device technologies, biotechnology, and mobile healthcare sectors through early-stage venture equity participation.
http://avetasystem.com
SOURCE Meditrina, Inc.
Related Links
https://www.meditrina-inc.com/
Horizon Technology Finance Provides $5 Million Venture Loan Facility to Meditrina
A leading specialty finance company that provides capital in the form of secured loans to venture capital-backed companies in the technology, life science, healthcare information and services, and cleantech industries, announced today it has provided a $5 million venture loan facility to Meditrina, Inc. ("Meditrina"), of which $3 million has been initially funded.
FARMINGTON, Conn., May 7, 2019 /PRNewswire/ -- Horizon Technology Finance Corporation (NASDAQ: HRZN) ("Horizon"), a leading specialty finance company that provides capital in the form of secured loans to venture capital-backed companies in the technology, life science, healthcare information and services, and cleantech industries, announced today it has provided a $5 million venture loan facility to Meditrina, Inc. ("Meditrina"), of which $3 million has been initially funded.
Meditrina is a pre-FDA cleared medical device company focused on developing fully disposable endoscopes and an office-based platform that can address multiple procedures for gynecologists. Meditrina's core management team and Board of Directors have collaborated on projects for two decades and achieved multiple successful exits in the gynecology space. Meditrina will use proceeds for general corporate purposes.
"Meditrina is developing efficient solutions for the diagnosis and treatment of a variety of minimally invasive uterine procedures that can be performed in the comfort of a doctor's office, which will be of significant benefit and convenience to patients and physicians," said Gerald A. Michaud, President of Horizon. "We are pleased to add Meditrina to our life sciences portfolio and help facilitate their next step in the FDA clearance process, and commercialization of the company's system, Aveta."
"We are excited to partner with Horizon as we continue to make progress in securing FDA clearance for our Aveta system," said Csaba Truckai, Founder of Meditrina, Inc. "Aveta is intended for hysteroscopic diagnostic and therapeutic uterine procedures and conveniently combines viewing, fluid management and resection capabilities all into one system. With Horizon's investment, we will be able to further our development and accelerate commercial activities of the Aveta system."
About Horizon Technology Finance
Horizon Technology Finance Corporation (NASDAQ: HRZN) is a leading specialty finance company that provides capital in the form of secured loans to venture capital backed companies in the technology, life science, healthcare information and services, and cleantech industries. The investment objective of Horizon is to maximize its investment portfolio's return by generating current income from the debt investments it makes and capital appreciation from the warrants it receives when making such debt investments. Headquartered in Farmington, Connecticut, Horizon also has regional offices in Pleasanton, California, Reston, Virginia and Boston, Massachusetts. To learn more, please visit www.horizontechfinance.com.
About Meditrina
Founded in 2016, Meditrina, Inc. is a pre-FDA cleared medical device company focused on developing fully disposable endoscopes and an all-inclusive office-based or clinical visualization platform that can address multiple procedures for gynecologists. Their first system, Aveta, is intended for hysteroscopic diagnostic and therapeutic uterine procedures (i.e. removal of polyps and fibroids). The Aveta system combines viewing, fluid management and resection capabilities into one system versus standalone systems in the market today. To learn more about Meditrina, please visit www.meditrina-inc.com.
Forward-Looking Statements
Statements included herein may constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Statements other than statements of historical facts included in this press release may constitute forward-looking statements and are not guarantees of future performance, condition or results and involve a number of risks and uncertainties. Actual results may differ materially from those in the forward-looking statements as a result of a number of factors, including those described from time to time in the Company's filings with the Securities and Exchange Commission. Horizon undertakes no duty to update any forward-looking statement made herein. All forward-looking statements speak only as of the date of this press release.
Contacts:
Investor Relations:
ICR
Garrett Edson
ir@horizontechfinance.com
(860) 284-6450
Media Relations:
ICR
Brian Ruby
brian.ruby@icrinc.com
(203) 682-8268
SOURCE Horizon Technology Finance Corporation
Related Links
http://www.horizontechfinance.com